IFNy in Primary HLH
IFNγ: a key cytokine in primary HLH1,2
IFNγ is a key cytokine in the immune system3,4
Interferon gamma (IFNγ) is the only type II interferon and plays an important role in cell communication during immune responses. During innate immune responses, IFNγ helps eliminate intracellular pathogens by activating macrophages and natural killer (NK) cells. During adaptive immune responses, IFNγ is responsible for both the differentiation and overproliferation of activated T cells.3,4
In primary hemophagocytic lymphohistiocytosis (HLH), the immune system is dysregulated and IFNγ contributes directly to disease pathogenesis.5
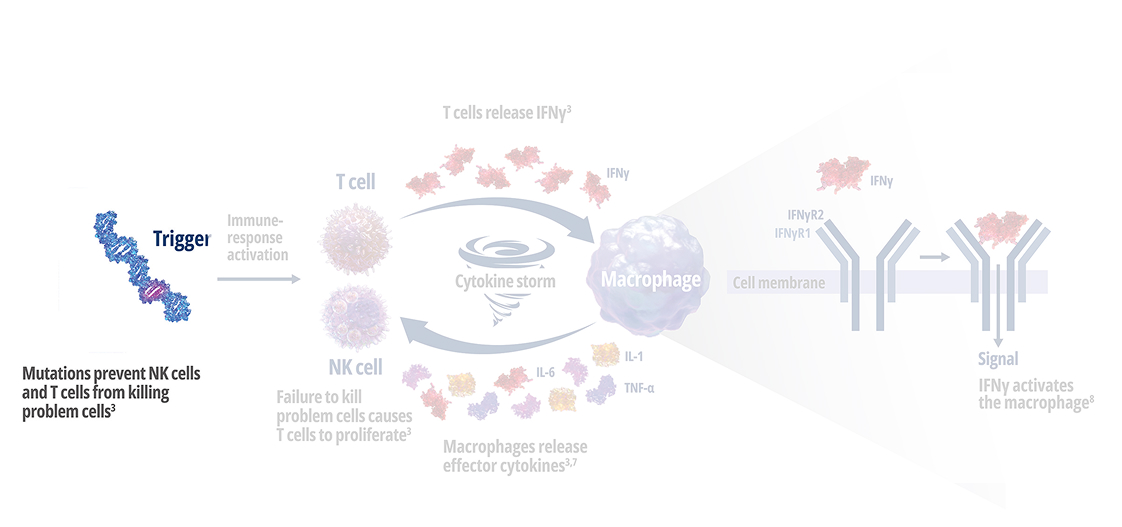
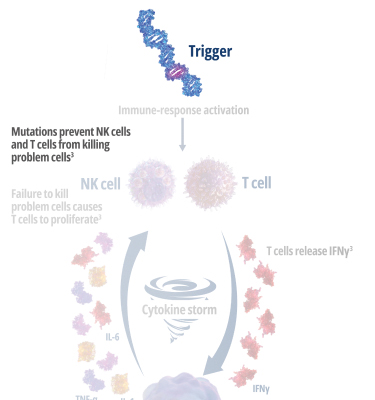
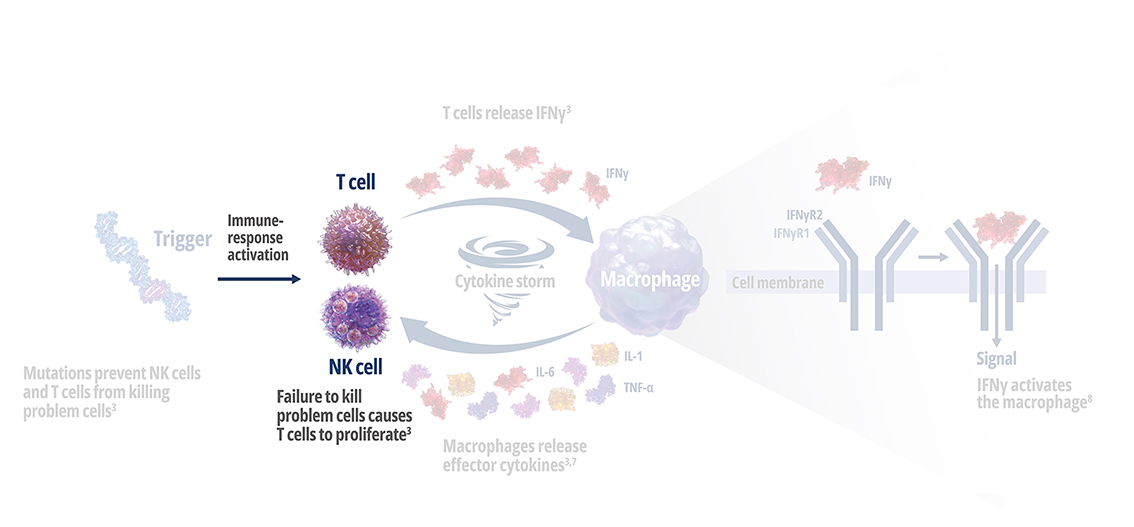
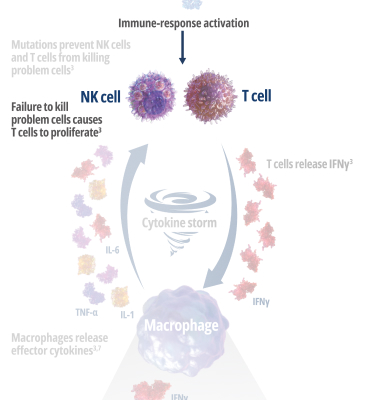
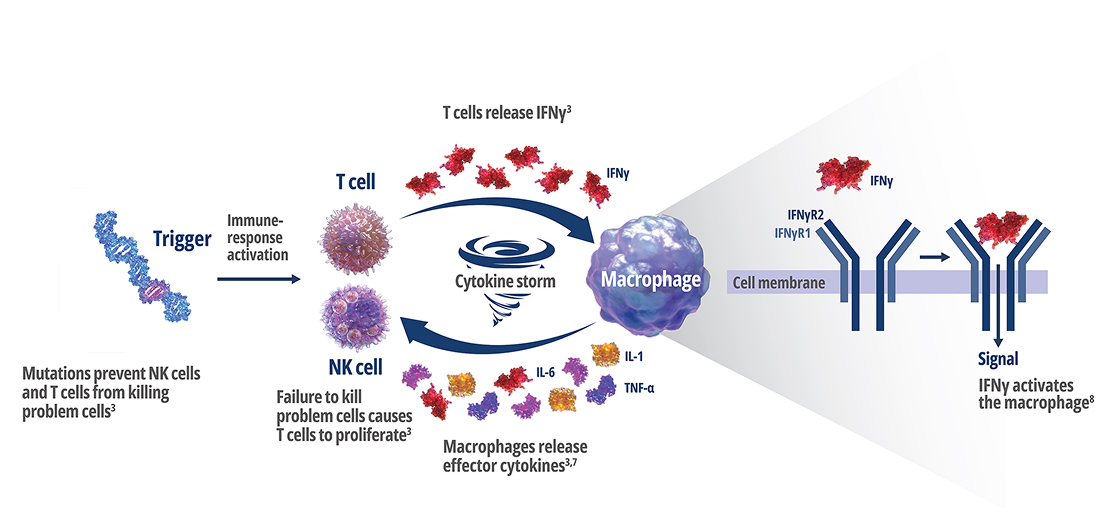
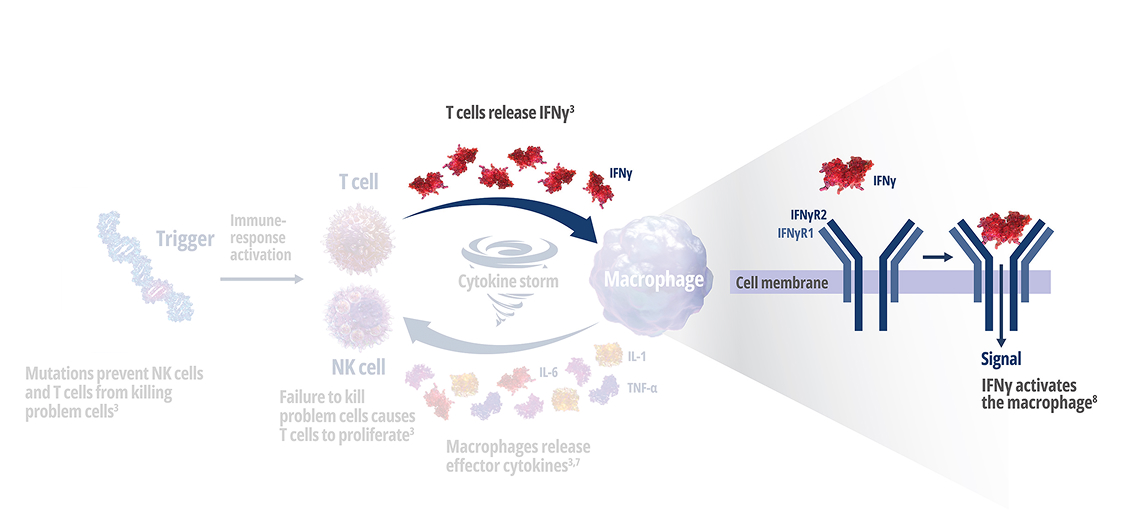
Genetic mutations disrupt immune function in primary HLH2
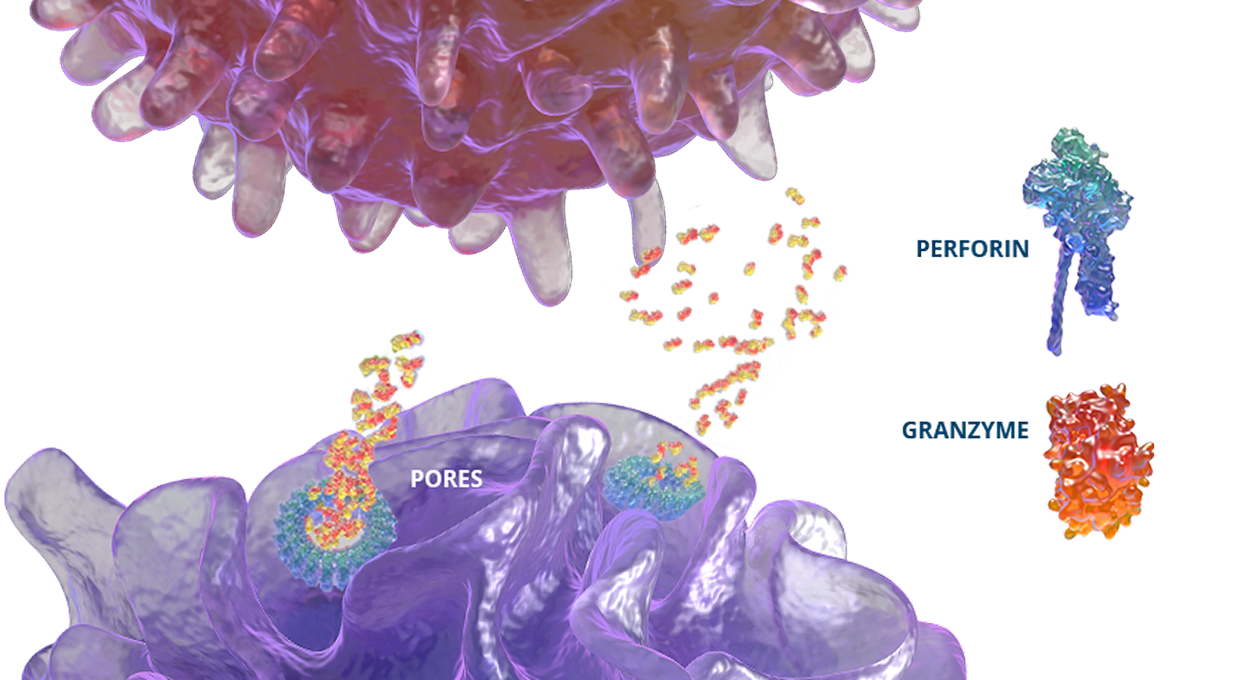
In healthy individuals, antigen-presenting cells (APCs) are recognized by cytotoxic CD8+ T cells, which bind to them to release perforin and granzymes into the immunological synapse space. Perforin creates pores in the target cell's plasma membrane, allowing the cytotoxic granzymes to enter and initiate lysis. In primary HLH, genetic mutations prevent perforin pore formation needed for cell lysis.7
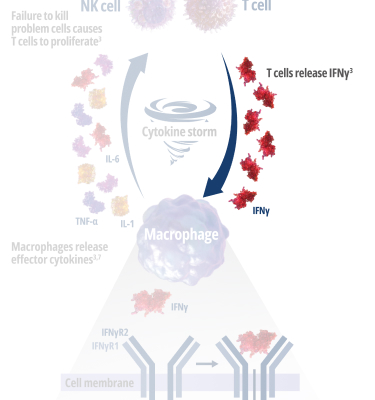
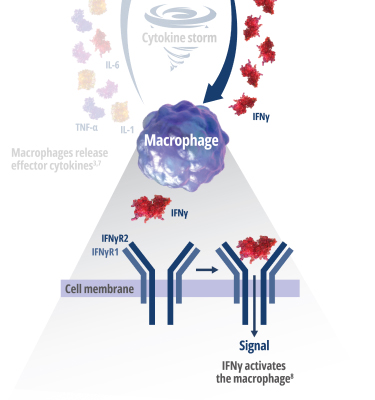
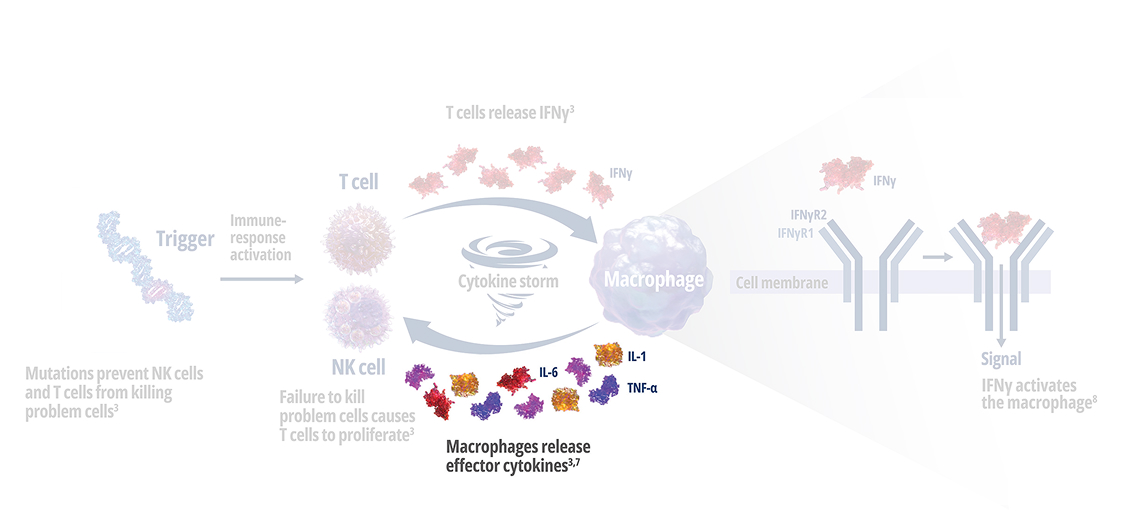
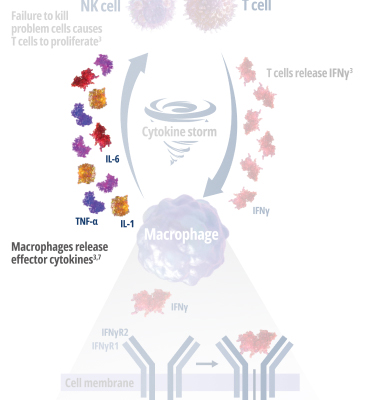
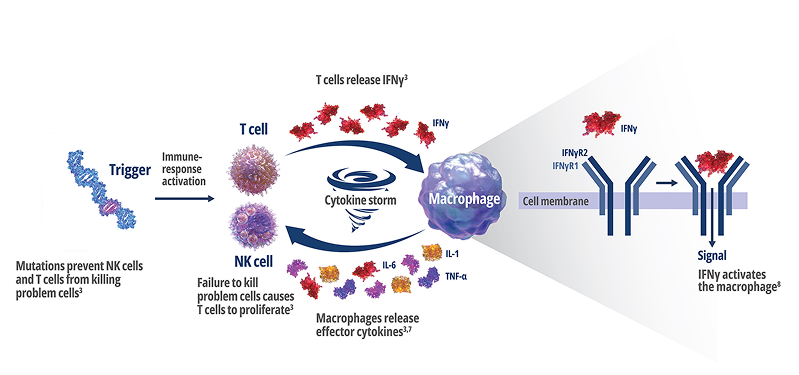
See how IFNγ activates a vicious cycle of hyperinflamation3
Click through or use the slider to see how IFNγ-activated macrophages trigger the downstream release of proinflammatory cytokines—including additional IFNγ—perpetuating hypercytokinemia and hyperinflammation in an aggressive continuum.3
IFNγR=interferon gamma receptor; IL=interleukin; TNF=tumor necrosis factor.
For more information about the critical role of IFNγ in primary HLH, download this guide.
Damaging effects of downstream cytokines
See how IFNγ triggers clinical and laboratory manifestations of disease.
Signs and symptoms of primary HLH | Drivers |
|---|---|
Fever9 |
|
Cytopenia10 |
|
Hypertriglyceridemia9,11 |
|
Hypofibrinogenemia12 |
|
Hyperferritinemia9,13 |
|
Elevated soluble CD25 (sCD25)14 |
|
Hepatosplenomegaly9 |
|
Elevated liver enzymes9 |
|
Hemophagocytosis15 |
|
The critical role of IFNγ in primary HLH
IFNγ was found to be essential for the development of HLH-like pathology. In murine models, inhibition of this cytokine led to an improvement of known features of HLH, including4,5:
- Increased blood cell counts (hemoglobin, platelets, and/or neutrophils)
- Significant reduction of triglyceride and ferritin levels
- Normalization of histopathological features of the spleen
- Reduction of macrophage activation, as evidenced by the reduction of hemophagocytosis in the liver